A2 Chemistry
Entropy is a measure of disorder. The universe tends towards disorder so if a reaction goes to a more ordered state work must be put in (this means the surroundings become less ordered).
Gases have a higher entropy than liquids, which have a higher entropy than solids. Entropy increases with temperature as particles move faster. Entropy also increases with the amount of substance, so entropy is higher when there are more moles. Entropy has important implications for Gibb’s Free Energy.
University Chemistry and Physics
Both Chemistry and Physics will expect you to know the entropy taught in A Level Chemistry.
Entropy is given the symbol S and it measured in JK-1mol-1. The official definition of entropy is ‘a system’s thermal energy per unit temperature available for doing useful work’. This is written as:
We saw in heat capacities that:

This can be substituted in and integrated:

In free expansion:
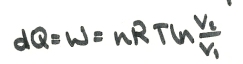
So:

For most liquids the entropy change when they are vaporised is around 85JK-1mol-1. This is called Trouton’s rule.
For spontaneous (irreversible change), the total change in entropy (including the system and surroundings) must be greater than zero. Reversible changes are only theoretical, and must have zero total entropy change. No process is possible in which the total entropy change is less than zero – this is the second law of thermodynamics.
It is impossible to calculate the entropy of a system, but easy to find the change in entropy.

